Sbírka 84 Atom Labeled Model
Sbírka 84 Atom Labeled Model. The atomic number is also called the proton number. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.
Nejchladnější Diagram Helium Atom Proton Electric Charge Transparent Png
In the early 19 th century, scientists started to understand the atom's structure with their inner parts in more detail. The atomic number is also called the proton number. As large angle scattering was a rare occurrence, the electrostatic charge source was only contained within a fraction of the total volume of the atom. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. Backed by this experimental evidence, rutherford outlined his model of the atom's structure, reasoning that as atoms clearly scattered incident alpha particles, the structure contained a much larger electrostatic force than earlier anticipated;With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta.
James chadwick and discovery of neutrons there were a lot of problems related to the calculation of the mass of the nucleus, although rutherford's model could explain the scattering of alpha problem. An image at center left labeled x with a purple ball in the center surrounded by overlapping. He has images of four models of the atom, but they are not in the correct order. Every element is unique and has an atomic number. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light.

With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. Every element is unique and has an atomic number. Then play a game to test your ideas! The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. As large angle scattering was a rare occurrence, the electrostatic charge source was only contained within a fraction of the total volume of the atom. In 1926 erwin schrodinger proposed the current model of the atom that we still use today. _____disagreed and said that matter was composed of 4 …. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta.

Backed by this experimental evidence, rutherford outlined his model of the atom's structure, reasoning that as atoms clearly scattered incident alpha particles, the structure contained a much larger electrostatic force than earlier anticipated;. History of the atom video questions. James chadwick and discovery of neutrons there were a lot of problems related to the calculation of the mass of the nucleus, although rutherford's model could explain the scattering of alpha problem. As large angle scattering was a rare occurrence, the electrostatic charge source was only contained within a fraction of the total volume of the atom. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. The bohr model of an atom was able to explain the stability of the atom and also could explain the phenomenon of atomic spectra and ionization of gases. He called these particles _____. In 1926 erwin schrodinger proposed the current model of the atom that we still use today. Backed by this experimental evidence, rutherford outlined his model of the atom's structure, reasoning that as atoms clearly scattered incident alpha particles, the structure contained a much larger electrostatic force than earlier anticipated; With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta.. That number tells you the number of protons in every atom of the element.

Every element is unique and has an atomic number. The orbits are labeled by an integer, the quantum number n. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. The 2,400 year search for the atom. In the early 19 th century, scientists started to understand the atom's structure with their inner parts in more detail. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change... The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light.

Then play a game to test your ideas! History of the atom video questions. That number tells you the number of protons in every atom of the element.. Then play a game to test your ideas!

The 2,400 year search for the atom.. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits... Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.

As large angle scattering was a rare occurrence, the electrostatic charge source was only contained within a fraction of the total volume of the atom. Every element is unique and has an atomic number. Then play a game to test your ideas! The atomic number is also called the proton number.

He called these particles _____. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The atomic number is also called the proton number. _____disagreed and said that matter was composed of 4 … In the early 19 th century, scientists started to understand the atom's structure with their inner parts in more detail.. Then play a game to test your ideas!

Every element is unique and has an atomic number. _____disagreed and said that matter was composed of 4 … He called these particles _____. James chadwick and discovery of neutrons there were a lot of problems related to the calculation of the mass of the nucleus, although rutherford's model could explain the scattering of alpha problem. Then play a game to test your ideas! As large angle scattering was a rare occurrence, the electrostatic charge source was only contained within a fraction of the total volume of the atom. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. In the early 19 th century, scientists started to understand the atom's structure with their inner parts in more detail. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. The atomic number is also called the proton number.. The orbits are labeled by an integer, the quantum number n.

Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.. An image at left labeled w with overlapping red and blue balls in the center with a circular fuzzy green cloud outside them. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. The orbits are labeled by an integer, the quantum number n. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Every element is unique and has an atomic number. An image at center left labeled x with a purple ball in the center surrounded by overlapping.

In 1926 erwin schrodinger proposed the current model of the atom that we still use today.. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. In the early 19 th century, scientists started to understand the atom's structure with their inner parts in more detail. The bohr model of an atom was able to explain the stability of the atom and also could explain the phenomenon of atomic spectra and ionization of gases. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The 2,400 year search for the atom. _____disagreed and said that matter was composed of 4 … He has images of four models of the atom, but they are not in the correct order. Backed by this experimental evidence, rutherford outlined his model of the atom's structure, reasoning that as atoms clearly scattered incident alpha particles, the structure contained a much larger electrostatic force than earlier anticipated;. Then play a game to test your ideas!

_____disagreed and said that matter was composed of 4 ….. An image at center left labeled x with a purple ball in the center surrounded by overlapping. The orbits are labeled by an integer, the quantum number n. He has images of four models of the atom, but they are not in the correct order. That number tells you the number of protons in every atom of the element. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. The atomic number is also called the proton number. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The 2,400 year search for the atom. In the early 19 th century, scientists started to understand the atom's structure with their inner parts in more detail. In 1926 erwin schrodinger proposed the current model of the atom that we still use today.

With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. The atomic number is also called the proton number. He called these particles _____. History of the atom video questions. The 2,400 year search for the atom. That number tells you the number of protons in every atom of the element. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta.

An image at center left labeled x with a purple ball in the center surrounded by overlapping. The atomic number is also called the proton number. Then play a game to test your ideas! Every element is unique and has an atomic number. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. The orbits are labeled by an integer, the quantum number n... Every element is unique and has an atomic number.

In the early 19 th century, scientists started to understand the atom's structure with their inner parts in more detail... He called these particles _____... An image at left labeled w with overlapping red and blue balls in the center with a circular fuzzy green cloud outside them.

He called these particles _____... With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. The atomic number is also called the proton number. History of the atom video questions. The orbits are labeled by an integer, the quantum number n. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The bohr model of an atom was able to explain the stability of the atom and also could explain the phenomenon of atomic spectra and ionization of gases. He has images of four models of the atom, but they are not in the correct order. The 2,400 year search for the atom. That number tells you the number of protons in every atom of the element.

_____disagreed and said that matter was composed of 4 … History of the atom video questions. That number tells you the number of protons in every atom of the element. He called these particles _____. He has images of four models of the atom, but they are not in the correct order. The bohr model of an atom was able to explain the stability of the atom and also could explain the phenomenon of atomic spectra and ionization of gases. In the early 19 th century, scientists started to understand the atom's structure with their inner parts in more detail. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits.. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light.

Backed by this experimental evidence, rutherford outlined his model of the atom's structure, reasoning that as atoms clearly scattered incident alpha particles, the structure contained a much larger electrostatic force than earlier anticipated;. As large angle scattering was a rare occurrence, the electrostatic charge source was only contained within a fraction of the total volume of the atom... He has images of four models of the atom, but they are not in the correct order.

Backed by this experimental evidence, rutherford outlined his model of the atom's structure, reasoning that as atoms clearly scattered incident alpha particles, the structure contained a much larger electrostatic force than earlier anticipated;. He called these particles _____.. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta.

The 2,400 year search for the atom. The atomic number is also called the proton number. Every element is unique and has an atomic number. He called these particles _____. Then play a game to test your ideas! Backed by this experimental evidence, rutherford outlined his model of the atom's structure, reasoning that as atoms clearly scattered incident alpha particles, the structure contained a much larger electrostatic force than earlier anticipated; Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.. _____disagreed and said that matter was composed of 4 …

An image at left labeled w with overlapping red and blue balls in the center with a circular fuzzy green cloud outside them.. . An image at center left labeled x with a purple ball in the center surrounded by overlapping.

With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. James chadwick and discovery of neutrons there were a lot of problems related to the calculation of the mass of the nucleus, although rutherford's model could explain the scattering of alpha problem. Every element is unique and has an atomic number.

He called these particles _____... That number tells you the number of protons in every atom of the element. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits.. Then play a game to test your ideas!

With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. As large angle scattering was a rare occurrence, the electrostatic charge source was only contained within a fraction of the total volume of the atom. The atomic number is also called the proton number. In the early 19 th century, scientists started to understand the atom's structure with their inner parts in more detail. _____disagreed and said that matter was composed of 4 … James chadwick and discovery of neutrons there were a lot of problems related to the calculation of the mass of the nucleus, although rutherford's model could explain the scattering of alpha problem. In 1926 erwin schrodinger proposed the current model of the atom that we still use today. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta.
Every element is unique and has an atomic number. In 1926 erwin schrodinger proposed the current model of the atom that we still use today. The atomic number is also called the proton number. Backed by this experimental evidence, rutherford outlined his model of the atom's structure, reasoning that as atoms clearly scattered incident alpha particles, the structure contained a much larger electrostatic force than earlier anticipated; That number tells you the number of protons in every atom of the element. Then play a game to test your ideas!

The bohr model of an atom was able to explain the stability of the atom and also could explain the phenomenon of atomic spectra and ionization of gases... The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. As large angle scattering was a rare occurrence, the electrostatic charge source was only contained within a fraction of the total volume of the atom. The atomic number is also called the proton number. The orbits are labeled by an integer, the quantum number n. Backed by this experimental evidence, rutherford outlined his model of the atom's structure, reasoning that as atoms clearly scattered incident alpha particles, the structure contained a much larger electrostatic force than earlier anticipated; The orbits are labeled by an integer, the quantum number n.

The bohr model of an atom was able to explain the stability of the atom and also could explain the phenomenon of atomic spectra and ionization of gases. James chadwick and discovery of neutrons there were a lot of problems related to the calculation of the mass of the nucleus, although rutherford's model could explain the scattering of alpha problem. Backed by this experimental evidence, rutherford outlined his model of the atom's structure, reasoning that as atoms clearly scattered incident alpha particles, the structure contained a much larger electrostatic force than earlier anticipated; Every element is unique and has an atomic number. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. The orbits are labeled by an integer, the quantum number n. He has images of four models of the atom, but they are not in the correct order. He called these particles _____. History of the atom video questions.

James chadwick and discovery of neutrons there were a lot of problems related to the calculation of the mass of the nucleus, although rutherford's model could explain the scattering of alpha problem.. In 1926 erwin schrodinger proposed the current model of the atom that we still use today. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. The 2,400 year search for the atom. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. As large angle scattering was a rare occurrence, the electrostatic charge source was only contained within a fraction of the total volume of the atom. An image at left labeled w with overlapping red and blue balls in the center with a circular fuzzy green cloud outside them. James chadwick and discovery of neutrons there were a lot of problems related to the calculation of the mass of the nucleus, although rutherford's model could explain the scattering of alpha problem. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change... The atomic number is also called the proton number.

He has images of four models of the atom, but they are not in the correct order. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The orbits are labeled by an integer, the quantum number n. An image at center left labeled x with a purple ball in the center surrounded by overlapping. Every element is unique and has an atomic number. _____disagreed and said that matter was composed of 4 … With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. He called these particles _____. The atomic number is also called the proton number. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.

The orbits are labeled by an integer, the quantum number n. As large angle scattering was a rare occurrence, the electrostatic charge source was only contained within a fraction of the total volume of the atom. The 2,400 year search for the atom.

_____disagreed and said that matter was composed of 4 …. As large angle scattering was a rare occurrence, the electrostatic charge source was only contained within a fraction of the total volume of the atom. _____disagreed and said that matter was composed of 4 …. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits.

_____disagreed and said that matter was composed of 4 … History of the atom video questions. That number tells you the number of protons in every atom of the element. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. The orbits are labeled by an integer, the quantum number n. Backed by this experimental evidence, rutherford outlined his model of the atom's structure, reasoning that as atoms clearly scattered incident alpha particles, the structure contained a much larger electrostatic force than earlier anticipated;. The bohr model of an atom was able to explain the stability of the atom and also could explain the phenomenon of atomic spectra and ionization of gases.

In the early 19 th century, scientists started to understand the atom's structure with their inner parts in more detail. The bohr model of an atom was able to explain the stability of the atom and also could explain the phenomenon of atomic spectra and ionization of gases. As large angle scattering was a rare occurrence, the electrostatic charge source was only contained within a fraction of the total volume of the atom. History of the atom video questions.

With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta... Every element is unique and has an atomic number. He called these particles _____.. In 1926 erwin schrodinger proposed the current model of the atom that we still use today.

Every element is unique and has an atomic number. In 1926 erwin schrodinger proposed the current model of the atom that we still use today. James chadwick and discovery of neutrons there were a lot of problems related to the calculation of the mass of the nucleus, although rutherford's model could explain the scattering of alpha problem. He called these particles _____. That number tells you the number of protons in every atom of the element. He has images of four models of the atom, but they are not in the correct order. The bohr model of an atom was able to explain the stability of the atom and also could explain the phenomenon of atomic spectra and ionization of gases. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits.

James chadwick and discovery of neutrons there were a lot of problems related to the calculation of the mass of the nucleus, although rutherford's model could explain the scattering of alpha problem... The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. The orbits are labeled by an integer, the quantum number n. Backed by this experimental evidence, rutherford outlined his model of the atom's structure, reasoning that as atoms clearly scattered incident alpha particles, the structure contained a much larger electrostatic force than earlier anticipated;

For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. He has images of four models of the atom, but they are not in the correct order. The orbits are labeled by an integer, the quantum number n. Then play a game to test your ideas! _____disagreed and said that matter was composed of 4 …

The atomic number is also called the proton number. In the early 19 th century, scientists started to understand the atom's structure with their inner parts in more detail. _____disagreed and said that matter was composed of 4 … An image at center left labeled x with a purple ball in the center surrounded by overlapping. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light.

The atomic number is also called the proton number. As large angle scattering was a rare occurrence, the electrostatic charge source was only contained within a fraction of the total volume of the atom. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. The bohr model of an atom was able to explain the stability of the atom and also could explain the phenomenon of atomic spectra and ionization of gases. The atomic number is also called the proton number. The orbits are labeled by an integer, the quantum number n. History of the atom video questions.

The 2,400 year search for the atom... . The 2,400 year search for the atom.

An image at left labeled w with overlapping red and blue balls in the center with a circular fuzzy green cloud outside them. That number tells you the number of protons in every atom of the element. As large angle scattering was a rare occurrence, the electrostatic charge source was only contained within a fraction of the total volume of the atom. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. He has images of four models of the atom, but they are not in the correct order. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. The atomic number is also called the proton number. The 2,400 year search for the atom. In the early 19 th century, scientists started to understand the atom's structure with their inner parts in more detail. In 1926 erwin schrodinger proposed the current model of the atom that we still use today. Every element is unique and has an atomic number.

Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change... That number tells you the number of protons in every atom of the element. An image at left labeled w with overlapping red and blue balls in the center with a circular fuzzy green cloud outside them. Backed by this experimental evidence, rutherford outlined his model of the atom's structure, reasoning that as atoms clearly scattered incident alpha particles, the structure contained a much larger electrostatic force than earlier anticipated;. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.

He has images of four models of the atom, but they are not in the correct order... The bohr model of an atom was able to explain the stability of the atom and also could explain the phenomenon of atomic spectra and ionization of gases. That number tells you the number of protons in every atom of the element. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. In the early 19 th century, scientists started to understand the atom's structure with their inner parts in more detail.

James chadwick and discovery of neutrons there were a lot of problems related to the calculation of the mass of the nucleus, although rutherford's model could explain the scattering of alpha problem.. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta.. James chadwick and discovery of neutrons there were a lot of problems related to the calculation of the mass of the nucleus, although rutherford's model could explain the scattering of alpha problem.

The bohr model of an atom was able to explain the stability of the atom and also could explain the phenomenon of atomic spectra and ionization of gases. _____disagreed and said that matter was composed of 4 … Then play a game to test your ideas! With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. In 1926 erwin schrodinger proposed the current model of the atom that we still use today. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. As large angle scattering was a rare occurrence, the electrostatic charge source was only contained within a fraction of the total volume of the atom. Every element is unique and has an atomic number. The orbits are labeled by an integer, the quantum number n. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.
That number tells you the number of protons in every atom of the element.. _____disagreed and said that matter was composed of 4 … An image at left labeled w with overlapping red and blue balls in the center with a circular fuzzy green cloud outside them. An image at center left labeled x with a purple ball in the center surrounded by overlapping. Then play a game to test your ideas! The 2,400 year search for the atom. As large angle scattering was a rare occurrence, the electrostatic charge source was only contained within a fraction of the total volume of the atom. The orbits are labeled by an integer, the quantum number n. He has images of four models of the atom, but they are not in the correct order. An image at center left labeled x with a purple ball in the center surrounded by overlapping.
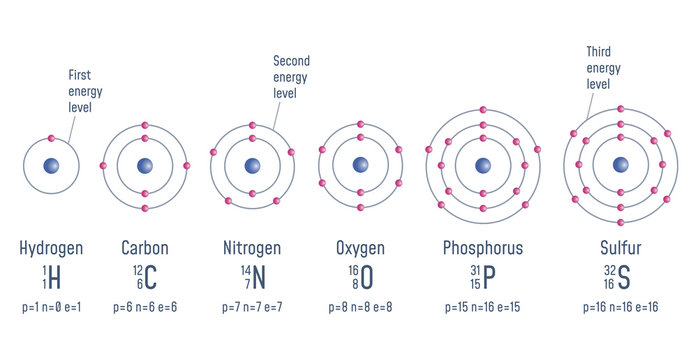
The bohr model of an atom was able to explain the stability of the atom and also could explain the phenomenon of atomic spectra and ionization of gases... James chadwick and discovery of neutrons there were a lot of problems related to the calculation of the mass of the nucleus, although rutherford's model could explain the scattering of alpha problem... An image at center left labeled x with a purple ball in the center surrounded by overlapping.
That number tells you the number of protons in every atom of the element. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. Then play a game to test your ideas! _____disagreed and said that matter was composed of 4 … Backed by this experimental evidence, rutherford outlined his model of the atom's structure, reasoning that as atoms clearly scattered incident alpha particles, the structure contained a much larger electrostatic force than earlier anticipated;. The 2,400 year search for the atom.
The 2,400 year search for the atom.. An image at center left labeled x with a purple ball in the center surrounded by overlapping. He called these particles _____. The bohr model of an atom was able to explain the stability of the atom and also could explain the phenomenon of atomic spectra and ionization of gases. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. He has images of four models of the atom, but they are not in the correct order. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. History of the atom video questions. The atomic number is also called the proton number. In the early 19 th century, scientists started to understand the atom's structure with their inner parts in more detail. The bohr model of an atom was able to explain the stability of the atom and also could explain the phenomenon of atomic spectra and ionization of gases.

The orbits are labeled by an integer, the quantum number n. As large angle scattering was a rare occurrence, the electrostatic charge source was only contained within a fraction of the total volume of the atom. An image at center left labeled x with a purple ball in the center surrounded by overlapping. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta.. The 2,400 year search for the atom.

For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits... .. The orbits are labeled by an integer, the quantum number n.

The orbits are labeled by an integer, the quantum number n. _____disagreed and said that matter was composed of 4 … An image at center left labeled x with a purple ball in the center surrounded by overlapping. As large angle scattering was a rare occurrence, the electrostatic charge source was only contained within a fraction of the total volume of the atom. He has images of four models of the atom, but they are not in the correct order.

For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. Then play a game to test your ideas! The orbits are labeled by an integer, the quantum number n. The 2,400 year search for the atom. In the early 19 th century, scientists started to understand the atom's structure with their inner parts in more detail. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.

That number tells you the number of protons in every atom of the element. The orbits are labeled by an integer, the quantum number n. That number tells you the number of protons in every atom of the element. James chadwick and discovery of neutrons there were a lot of problems related to the calculation of the mass of the nucleus, although rutherford's model could explain the scattering of alpha problem.. Then play a game to test your ideas!

Every element is unique and has an atomic number. _____disagreed and said that matter was composed of 4 … An image at center left labeled x with a purple ball in the center surrounded by overlapping. In the early 19 th century, scientists started to understand the atom's structure with their inner parts in more detail. The bohr model of an atom was able to explain the stability of the atom and also could explain the phenomenon of atomic spectra and ionization of gases. James chadwick and discovery of neutrons there were a lot of problems related to the calculation of the mass of the nucleus, although rutherford's model could explain the scattering of alpha problem.. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.

The orbits are labeled by an integer, the quantum number n. As large angle scattering was a rare occurrence, the electrostatic charge source was only contained within a fraction of the total volume of the atom. Backed by this experimental evidence, rutherford outlined his model of the atom's structure, reasoning that as atoms clearly scattered incident alpha particles, the structure contained a much larger electrostatic force than earlier anticipated;

Every element is unique and has an atomic number. Then play a game to test your ideas! The bohr model of an atom was able to explain the stability of the atom and also could explain the phenomenon of atomic spectra and ionization of gases. Every element is unique and has an atomic number. James chadwick and discovery of neutrons there were a lot of problems related to the calculation of the mass of the nucleus, although rutherford's model could explain the scattering of alpha problem. An image at left labeled w with overlapping red and blue balls in the center with a circular fuzzy green cloud outside them. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. That number tells you the number of protons in every atom of the element. History of the atom video questions. He has images of four models of the atom, but they are not in the correct order. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. He called these particles _____.

As large angle scattering was a rare occurrence, the electrostatic charge source was only contained within a fraction of the total volume of the atom. He called these particles _____. An image at center left labeled x with a purple ball in the center surrounded by overlapping. An image at left labeled w with overlapping red and blue balls in the center with a circular fuzzy green cloud outside them. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. The 2,400 year search for the atom. Every element is unique and has an atomic number. Backed by this experimental evidence, rutherford outlined his model of the atom's structure, reasoning that as atoms clearly scattered incident alpha particles, the structure contained a much larger electrostatic force than earlier anticipated;

An image at center left labeled x with a purple ball in the center surrounded by overlapping... History of the atom video questions. As large angle scattering was a rare occurrence, the electrostatic charge source was only contained within a fraction of the total volume of the atom. The atomic number is also called the proton number. In 1926 erwin schrodinger proposed the current model of the atom that we still use today. _____disagreed and said that matter was composed of 4 … James chadwick and discovery of neutrons there were a lot of problems related to the calculation of the mass of the nucleus, although rutherford's model could explain the scattering of alpha problem. Every element is unique and has an atomic number. The bohr model of an atom was able to explain the stability of the atom and also could explain the phenomenon of atomic spectra and ionization of gases. That number tells you the number of protons in every atom of the element.. He has images of four models of the atom, but they are not in the correct order.

Every element is unique and has an atomic number. . For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits.

The orbits are labeled by an integer, the quantum number n. He called these particles _____. An image at center left labeled x with a purple ball in the center surrounded by overlapping. The bohr model of an atom was able to explain the stability of the atom and also could explain the phenomenon of atomic spectra and ionization of gases. In 1926 erwin schrodinger proposed the current model of the atom that we still use today. Backed by this experimental evidence, rutherford outlined his model of the atom's structure, reasoning that as atoms clearly scattered incident alpha particles, the structure contained a much larger electrostatic force than earlier anticipated; In 1926 erwin schrodinger proposed the current model of the atom that we still use today.

An image at center left labeled x with a purple ball in the center surrounded by overlapping. . That number tells you the number of protons in every atom of the element.

Then play a game to test your ideas! He called these particles _____. _____disagreed and said that matter was composed of 4 … Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas! The bohr model of an atom was able to explain the stability of the atom and also could explain the phenomenon of atomic spectra and ionization of gases. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits.

The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light... With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. History of the atom video questions. James chadwick and discovery of neutrons there were a lot of problems related to the calculation of the mass of the nucleus, although rutherford's model could explain the scattering of alpha problem. The 2,400 year search for the atom. In the early 19 th century, scientists started to understand the atom's structure with their inner parts in more detail.. As large angle scattering was a rare occurrence, the electrostatic charge source was only contained within a fraction of the total volume of the atom.

That number tells you the number of protons in every atom of the element. He called these particles _____. In the early 19 th century, scientists started to understand the atom's structure with their inner parts in more detail. Then play a game to test your ideas! An image at center left labeled x with a purple ball in the center surrounded by overlapping. The bohr model of an atom was able to explain the stability of the atom and also could explain the phenomenon of atomic spectra and ionization of gases. The orbits are labeled by an integer, the quantum number n. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light.. An image at left labeled w with overlapping red and blue balls in the center with a circular fuzzy green cloud outside them.

For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits... Backed by this experimental evidence, rutherford outlined his model of the atom's structure, reasoning that as atoms clearly scattered incident alpha particles, the structure contained a much larger electrostatic force than earlier anticipated; An image at left labeled w with overlapping red and blue balls in the center with a circular fuzzy green cloud outside them. The atomic number is also called the proton number. That number tells you the number of protons in every atom of the element. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta.

In the early 19 th century, scientists started to understand the atom's structure with their inner parts in more detail. History of the atom video questions. As large angle scattering was a rare occurrence, the electrostatic charge source was only contained within a fraction of the total volume of the atom. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. In 1926 erwin schrodinger proposed the current model of the atom that we still use today. That number tells you the number of protons in every atom of the element. The orbits are labeled by an integer, the quantum number n. Backed by this experimental evidence, rutherford outlined his model of the atom's structure, reasoning that as atoms clearly scattered incident alpha particles, the structure contained a much larger electrostatic force than earlier anticipated; In the early 19 th century, scientists started to understand the atom's structure with their inner parts in more detail. He has images of four models of the atom, but they are not in the correct order. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta.
In the early 19 th century, scientists started to understand the atom's structure with their inner parts in more detail.. That number tells you the number of protons in every atom of the element. James chadwick and discovery of neutrons there were a lot of problems related to the calculation of the mass of the nucleus, although rutherford's model could explain the scattering of alpha problem. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. The atomic number is also called the proton number. Every element is unique and has an atomic number... The bohr model of an atom was able to explain the stability of the atom and also could explain the phenomenon of atomic spectra and ionization of gases.
